Lithium Concentrations in Groundwater Used as Drinking Water for the Conterminous United States. [View all]
The paper to which I'll refer in this post is this one: Estimating Lithium Concentrations in Groundwater Used as Drinking Water for the Conterminous United States Melissa A. Lombard, Eric E. Brown, Daniel M. Saftner, Monica M. Arienzo, Esme Fuller-Thomson, Craig J. Brown, and Joseph D. Ayotte Environmental Science & Technology 2024 58 (2), 1255-1264
The article is open access for the public to read, nevertheless I'll offer a brief excerpt for convenience.
From the introduction of the paper:
Lithium (Li) is a naturally occurring alkali metal found in minerals and in groundwater and surface water as a monovalent cation. Li concentrations in drinking-water supplies are not currently regulated in the United States; therefore, its occurrence has not been commonly measured. Li is used as a medication to treat bipolar disorder and depression, (1) and clinical doses typically range between 600 and 1800 mg per day, which is 2–3 orders of magnitude greater than typical drinking-water concentrations (less than 0.030 mg/L). (2) Studies have linked the low-level occurrence (0.1–219 μg/L) of Li in drinking water to positive human-health outcomes such as reduced suicide mortality (3−7) and other mental-health benefits (2,8−11) in addition to potential negative outcomes such as autism (12) and thyroid hormone levels. (13,14) Further, side effects impacting renal, neurological, dermal, cardiovascular, and endocrine systems can occur due to Li used clinically, especially at higher doses (2.74–4.2 mg Li/kg of body weight per day). (15) The U.S. Environmental Protection Agency (EPA) established a provisional reference dose (p-RfD) of 2 μg of Li per kilogram of body weight per day and reports that confidence in this value is low to medium because of a lack of dose–response information at subclinical concentrations. (15) The U.S. Geological Survey developed a nonenforceable health-based screening level of 10 μg/L for Li in drinking water based on the p-RfD. (16) Li is included in the most recent EPA list of unregulated contaminants to be monitored by public water systems as part of the fifth Unregulated Contaminant Monitoring Rule (UCMR5). This rule requires public water suppliers to measure Li concentrations in drinking water starting in 2023 to provide nationally distributed data on its occurrence. (17) The inclusion of Li in UCMR5 indicates the potential for future regulations in public drinking-water supply utilities.]
A graphic from the paper:
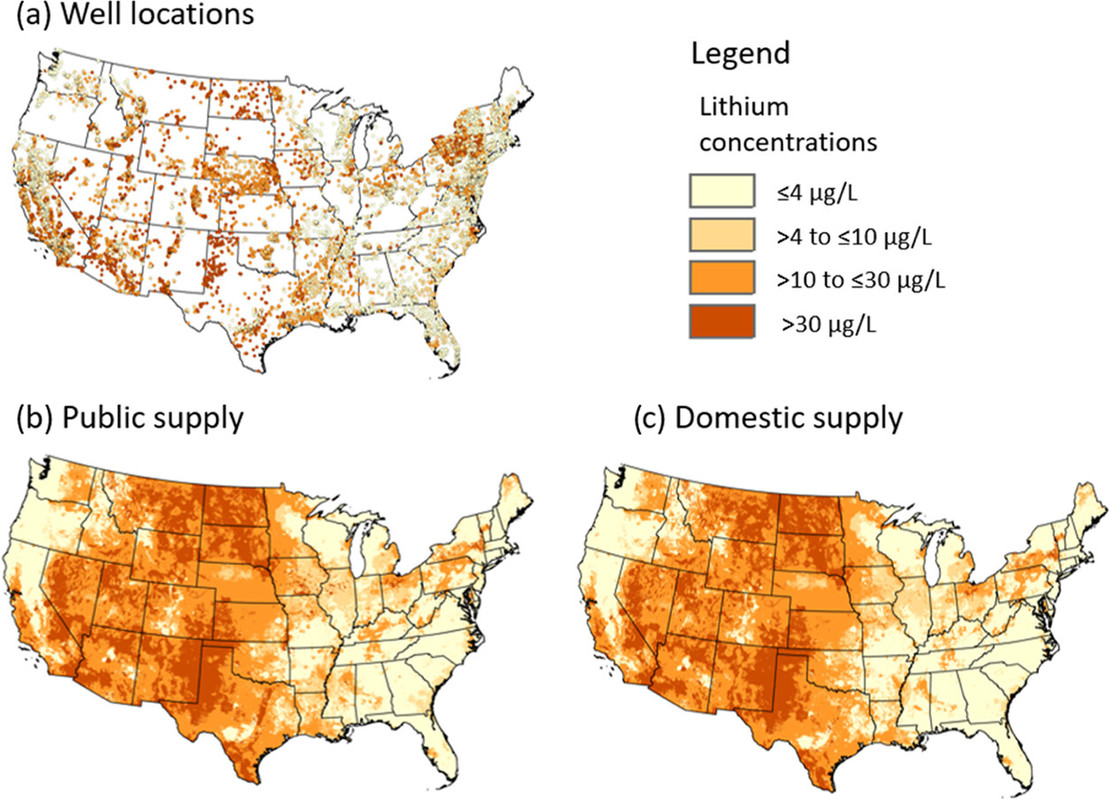
The caption:
Figure 1. Lithium concentrations in groundwater (a) at well locations, (b) modeled for public-supply well depths, and (c) modeled for private-supply well depths.
No comment, other than that figure 6 in the paper, if one opens it, is interesting.
