Science
Related: About this forumPerplexing diamonds from South Africa mine contain 'almost impossible' chemistry
By Stephanie Pappas published 2 days ago
Seemingly contradictory materials are trapped together in two glittering diamonds from South Africa, shedding light on how diamonds form.
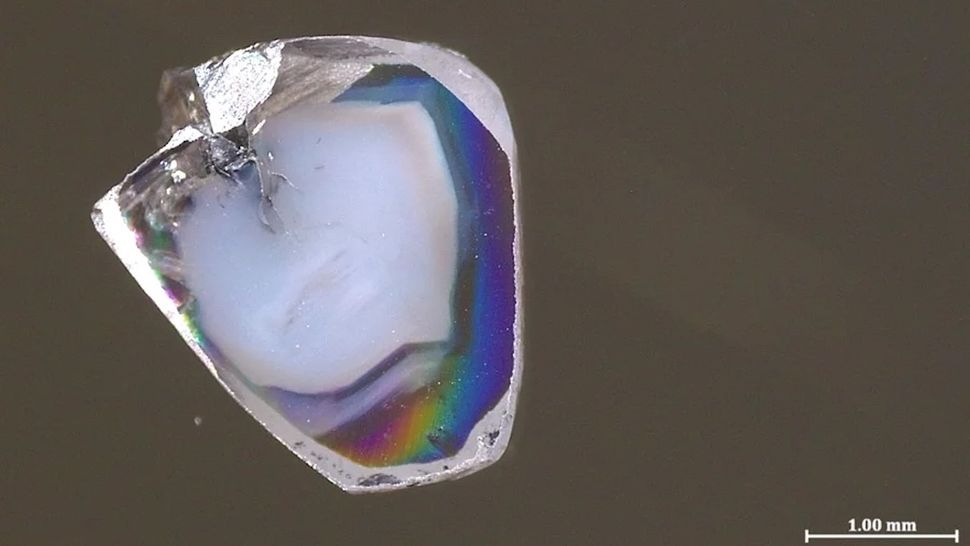
A close-up of a small diamond fragment
Deep-Earth diamond. (Image credit: Yael Kempe and Yakov Weiss)
A pair of diamonds that formed hundreds of kilometers deep in Earth's malleable mantle both contain specks of materials that form in completely opposing chemical environments — a combination so unusual that researchers thought their coexistence was "almost impossible." The substances' presence provides a window into the chemical goings-on of the mantle and the reactions that form diamonds.
The two diamond samples were found in a South African mine. As with plenty of other precious gemstones, they contain what are called inclusions — tiny bits of surrounding rocks captured as the diamonds form. These inclusions are loathed by most jewelers but are an exciting source of information for scientists. That's especially true when diamonds form deep in the unreachable mantle, because they carry these inclusions basically undisturbed to the surface — the only way those minerals can rise hundreds of kilometers without being altered from their original deep-mantle state.
The two new diamond samples each contain inclusions of carbonate minerals that are rich in oxygen atoms (a state known as oxidized) and oxygen-poor nickel alloys (a state known as reduced, in the parlance of chemistry). Much like how an acid and a base immediately react to form water and a salt, oxidized carbonate minerals and reduced metals don't coexist for long. Typically, diamond inclusions show just one or the other, so the presence of both perplexed Yaakov Weiss, a senior lecturer in Earth sciences at the Hebrew University of Jerusalem, and his colleagues — so much so that they initially put the samples aside for a year in confusion, he says.
But when they reanalyzed the diamonds, the researchers realized that the inclusions capture a snapshot of the reaction that made the sparkling stones and confirm for the first time that diamonds can form when carbonate minerals and reduced metals in the mantle react. The new samples are the first time scientists have ever seen the midpoint of that reaction captured in a natural diamond.
More:
https://www.livescience.com/planet-earth/geology/perplexing-diamonds-from-south-africa-mine-contain-almost-impossible-chemistry
Ilsa
(63,333 posts)Thank you. It's a peek into a process never imagined!