Environment & Energy
Related: About this forumEfforts to Reduce the High Energy, Carbon and Chemical Waste Cost of Recycling Lithium Ion Batteries.
The paper I'll briefly discuss in this post is this one: Three-in-One Self-Sacrifice Mechanism Driving Green and Universal Critical Metals Leaching from Spent Lithium-Ion Batteries Xiaonan Feng, Xiaosong Gu, Qiang Zeng, Xuezhen Feng, Yangzi Shangguan, Jiaxin Liang, Jiaxiang Liang, Weixu Zhong, Hao Fan, Qi Yue, Ranhao Wang, and Hong Chen Environmental Science & Technology 2025 59 (45), 24380-24391
The big lie we hear around here from time to time is that energy storage is "green" because allegedly, although not in practice, so called "renewable energy" is "green," even if we address the appalling lack of reliability by storing the energy generated when its not needed.
On inspection, this is nonsense. The land and material costs of so called "renewable energy" are unsustainable, and the use of batteries, concomitant with their material and mining implications, wastes energy, as the inviolable laws of thermodynamics unambiguously indicate.
Batteries themselves have a huge environmental impact; the chemistry is not clean, as the article shows, even with magical "recycling" because recycling isn't "green."
From the article's introduction:
Cathodes with high-density critical metals are the most valuable component in spent LIBs. (6) To recycle critical metals from the spent cathode, pyrometallurgy and hydrometallurgy methods are extensively employed for the recycling of spent LIBs. (7) Meanwhile, the pyrometallurgy approach requires a pyrolysis treatment at high temperatures, resulting in higher energy consumption, severe CO2 emissions, and secondary pollution. (8) In contrast, the hydrometallurgical process, which involves massive amounts of hazardous acid or potent redox agents shipped long distances and then used in wet-chemistry leaching, has been extensively employed in spent cathode recycling. (9−11) Despite the high leaching efficiency, the hazardous nature of acid or oxidizing agents may cause substantial adverse environmental and health effects, resulting in excess wastewater or air pollution and leading to severe health and safety issues (Figure 1a). (12−14) In this regard, developing green and environmentally friendly solvents for the universal and sustainable leaching of critical metals from LIBs is urgently needed. Under these circumstances, mild and green extraction solvents such as hydrogen peroxide (H2O2), sodium sulfite (Na2SO3), persulfate, and deep-eutectic solvents (DES) have been recognized as greener reagents, recently being explored for cathode leaching. (15,16) For instance, a deep eutectic solvent composed of chloroacetic acid and ethanol, with oxygen as the oxidant, has been applied for the recovery of lithium from spent LiFePO4 (LFP) powder. (17) In addition, sulfites and sodium persulfate have been employed as oxidants to selectively extract lithium from spent LiCoO2 (LCO) and LiMn2O4 (LMO), respectively. (18,19) Unfortunately, their high cost and low universality hinder their broad application for recycling diverse types of spent LIB cathodes.
Herein, to develop a green and universal agent for critical metal recycling from multiple types of LIBs cathodes, we employed a multifunctional peracetic acid (PAA) for highly efficient critical metal extraction from different spent cathodes (Figure 1a). Benefiting from the high redox potentials, (20) acidic proton attacking, and coordination function (denoted as three-in-one driven forces) from the carboxyl group, PAA undergoes a self-sacrifice process and delivers a highly efficient critical metal recovery from multiple types of cathodes, including LFP, LCO, LMO, and LiNixCoyMn1–x–yO[sub[2 (NCM). Furthermore, acetic acid (HAc) could be recovered from wastewater after critical metal precipitation, thereby enabling solvent recycling and contributing to a closed-loop wastewater treatment process. Employing PAA as the green critical metal extraction reagent here demonstrates a novel, integrated three-in-one self-sacrifice mechanism for universal critical metal leaching, which is technically important for the circular economy and closed-loop resource utilization of urban mines.
We're saved.
(The next paper in this issue questions whether there is really enough metal to mine on the planet to make all this magical batteries, but I won't have time to go there tonight, or maybe even at any point in the future.)
Some figures from the paper:
The first refers to the state of the art and the proposed modification:
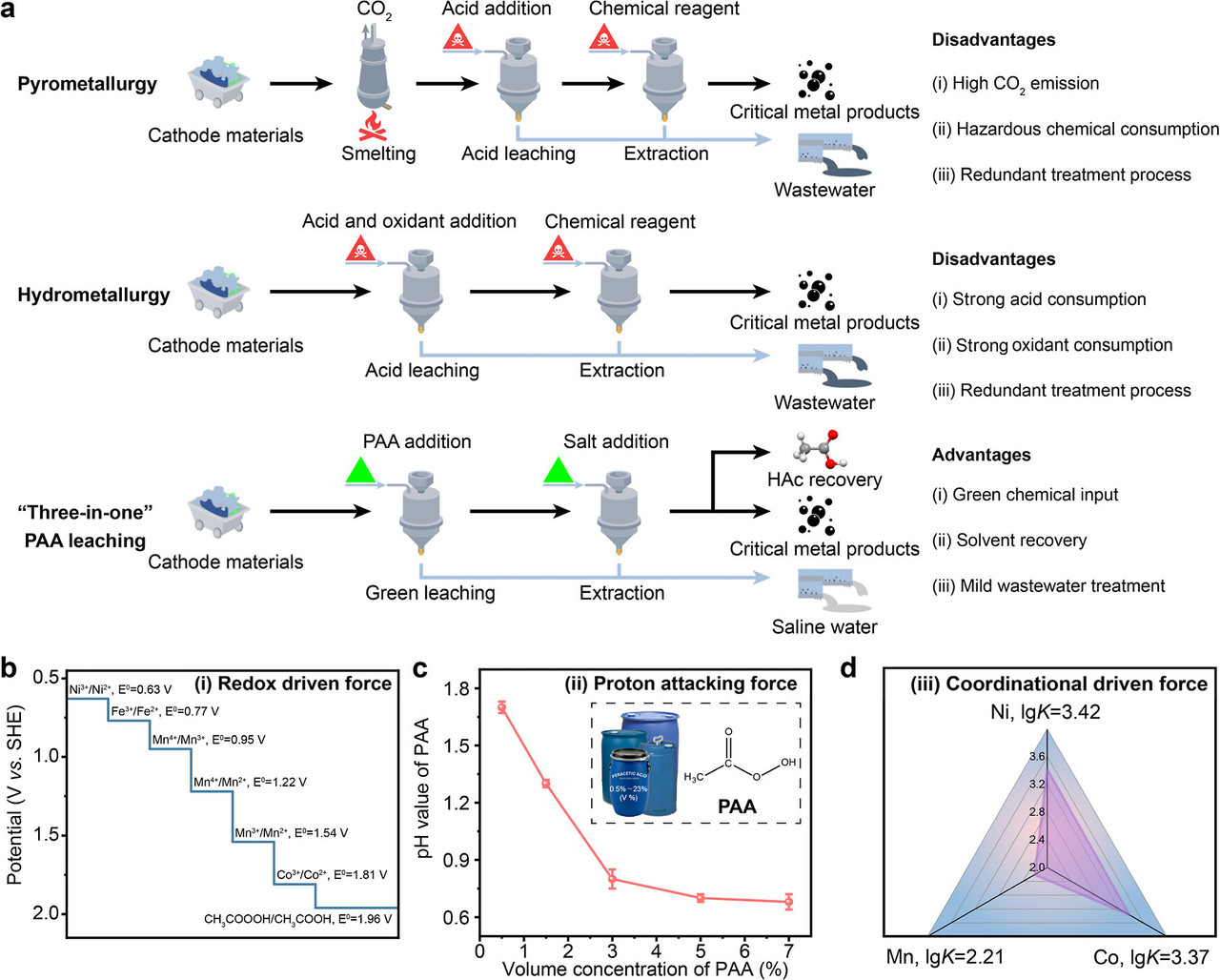
The caption:
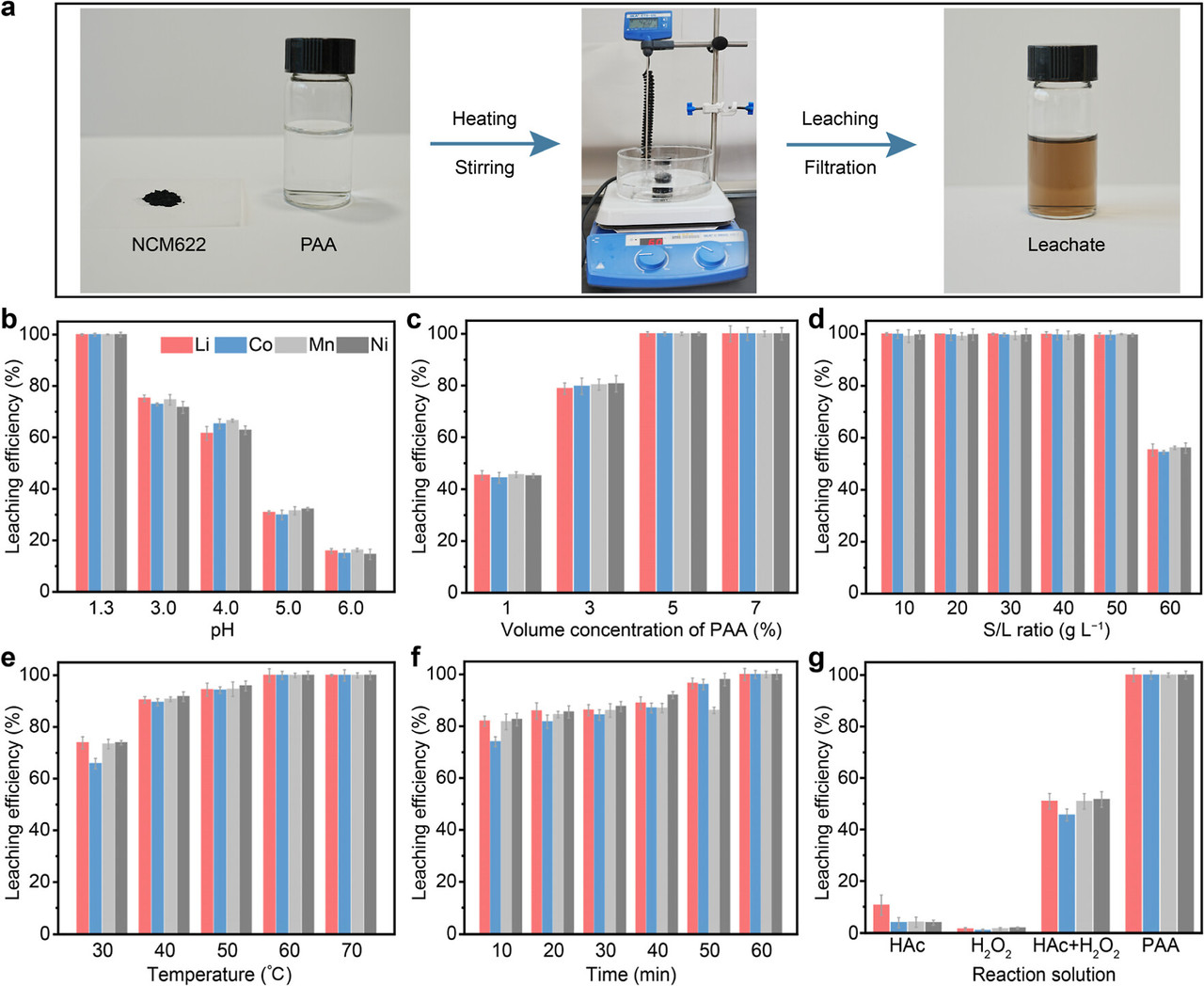
The caption:
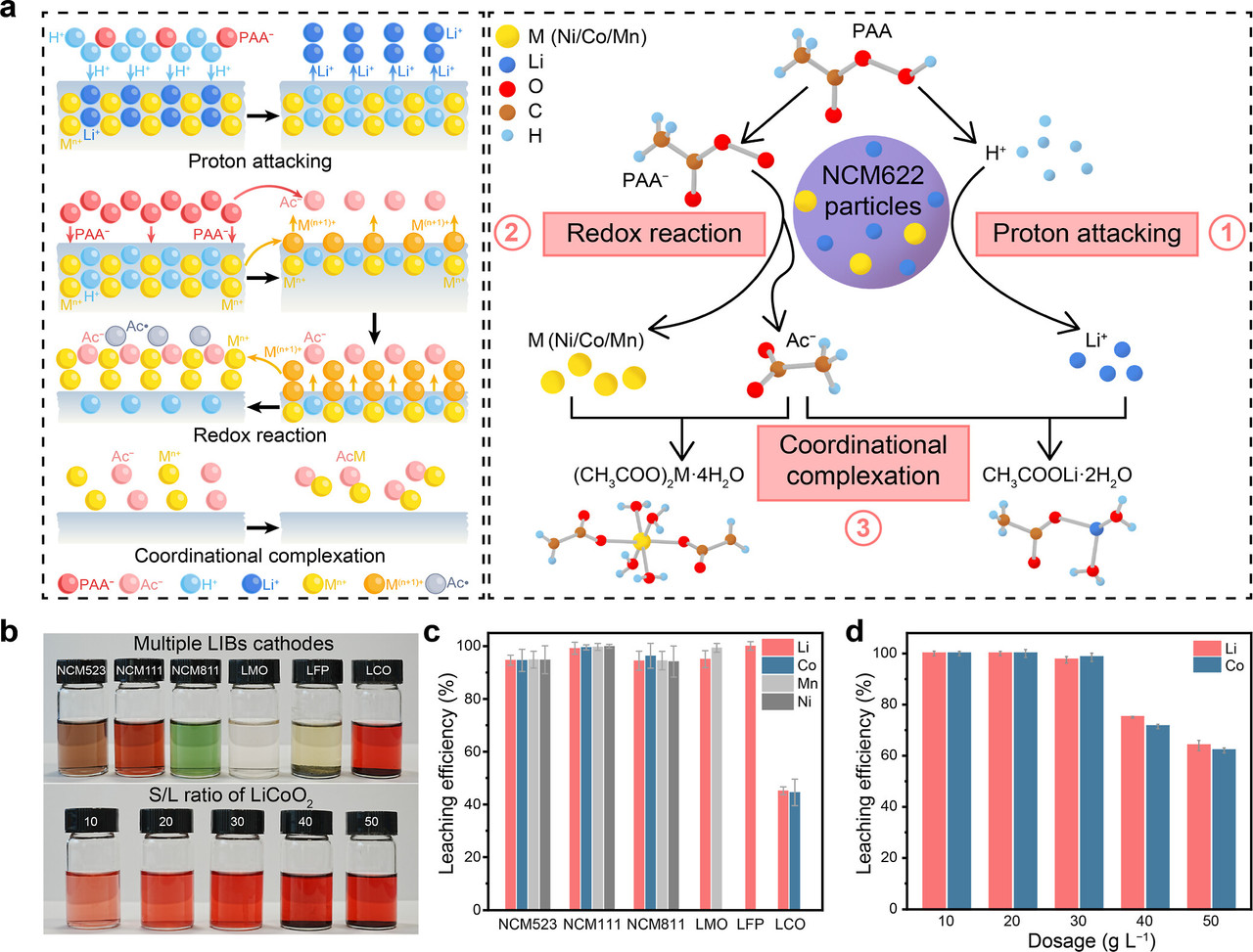
The caption:
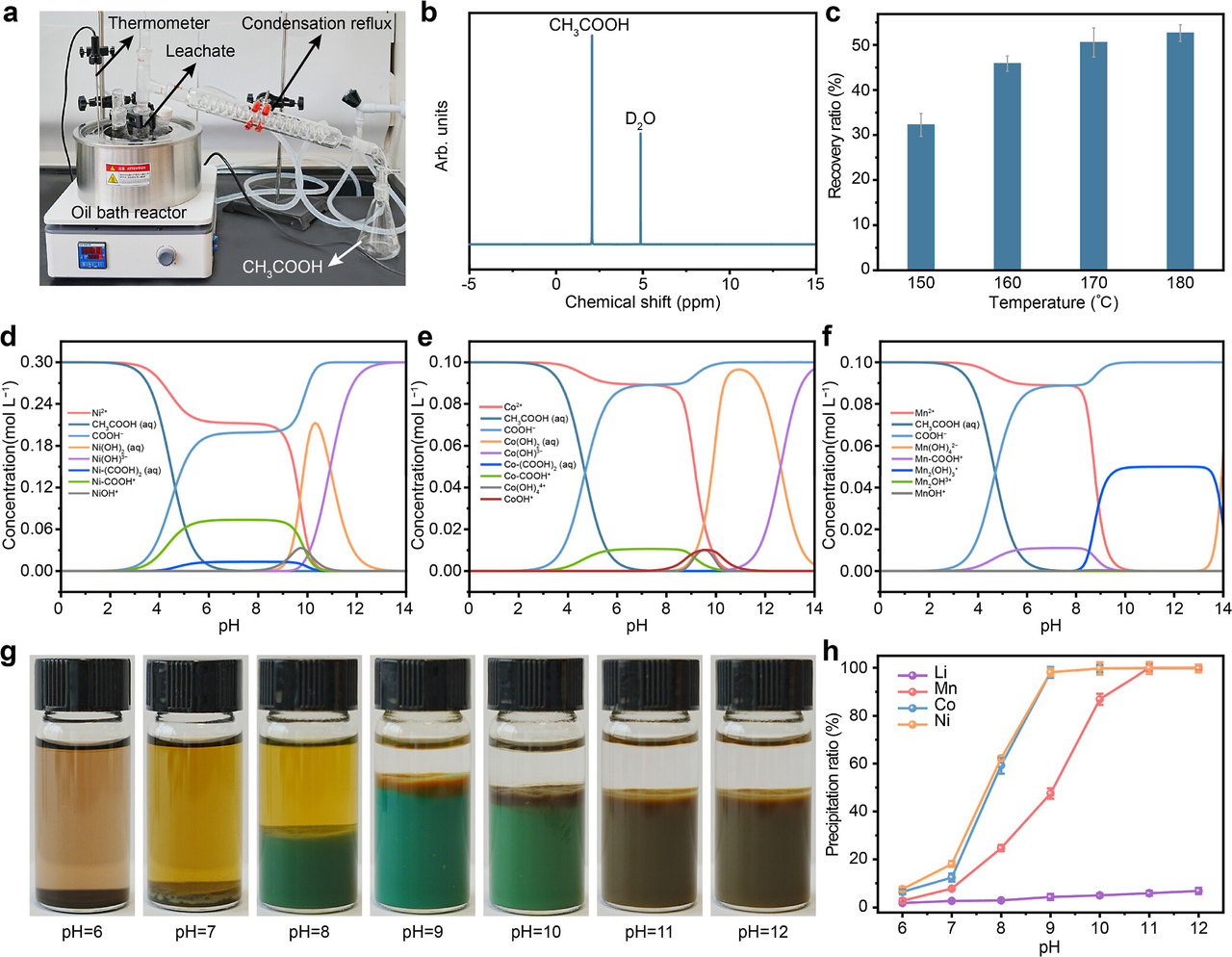
The caption:
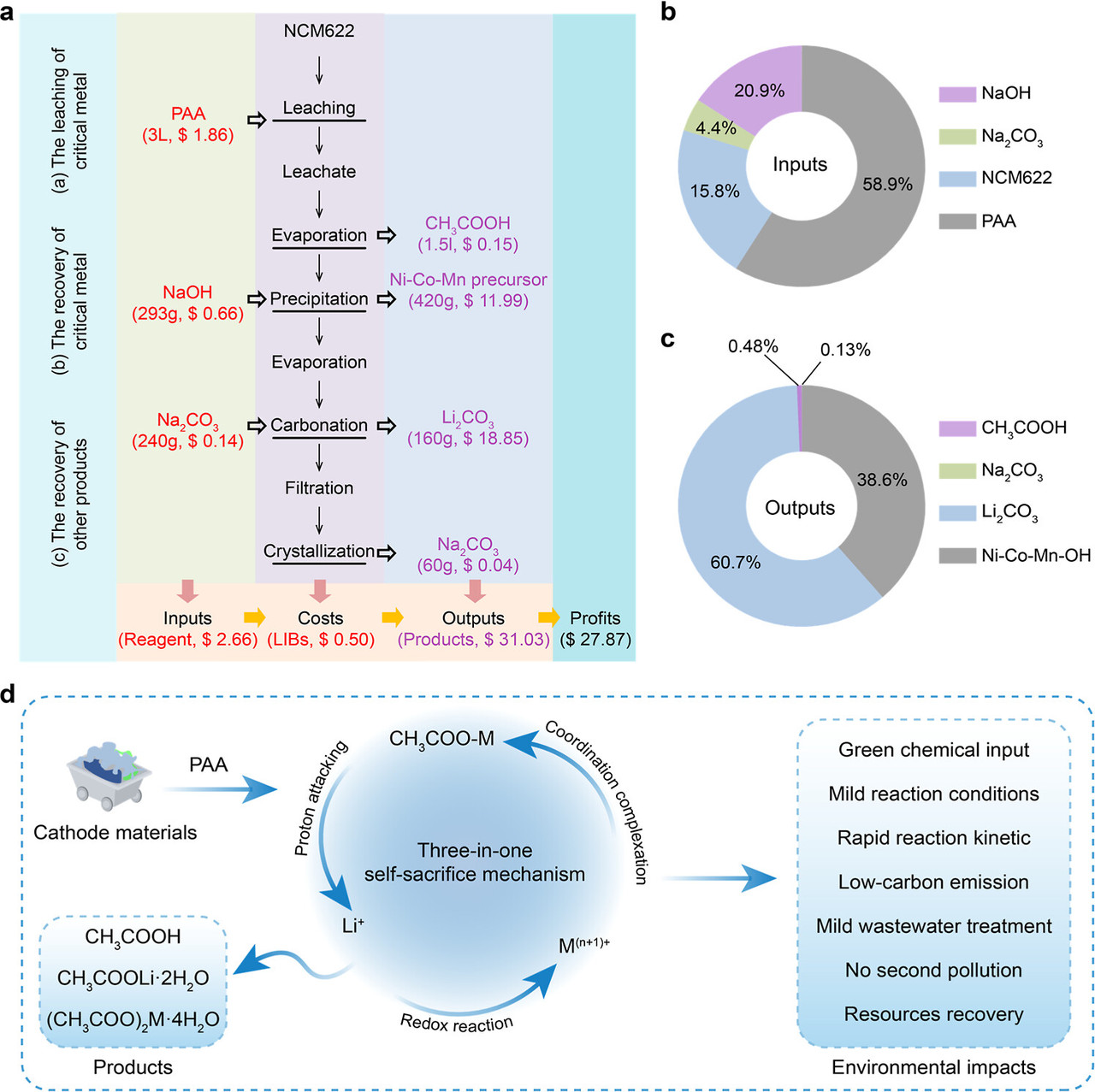
The caption:
The author's offer up their concluding claims:
We're saved, especially if we can find lots of poor people to run these processes.
Have a nice day tomorrow.