Environment & Energy
Related: About this forumEcological Risk of Perfluorooctanoic Acid (PFOA) Degradation Products.
The paper to which I'll refer in this post is this one: Enhanced Ecological Risk of PFOA Degradation Products: Insights from Concentration-Dependent Transcriptomics, Adverse Outcome Pathways, and Biomarker Verification Mingyang Li, Xiao Gou, Chao Zhang, Xiaowei Zhang, and Wei Jiang Environmental Science & Technology 2025 59 (26), 13131-13142.
Among the most intractable pollutants are the fluorinated alkanes, of which many hundreds, if not thousands, have been produced industrially. One of the most prominent of these is perfluorooctanoic acid (PFOA), a constituent of many commercial products, in particular, those sold to protect furniture from staining, "scotch guard" being such a product. (You may have sprayed on your furniture at some point in your life. If so, it's still there.)
The carbon fluorine bond is one of the strongest in chemistry, having an energy of about 490 kJ/mol, which, as one can calculate from high school level quantum mechanics, puts the radiation required for bond scission in the UV range, at a frequency of about 1.2 X 1015 sec-1, well into the UV range. I have often suggested to my son that this suggests that one among many valuable components of used nuclear fuel, the fission product 137Cs, which exists in secular equilibrium with the powerful gamma emitter, 137mBa, would represent a wonderful tool for the degradation of PFOA and other fluorochemicals.
An issue, discussed in the paper is however, that the mineralization should be complete, since the intermediate degradants are problematic themselves.
From the introductory text:
The authors subject PFOA solutions in water to 300 nm (UV) radiation to produce the shorter chain perfluoroacids and then expose them to various test species.
Some figures from the text:
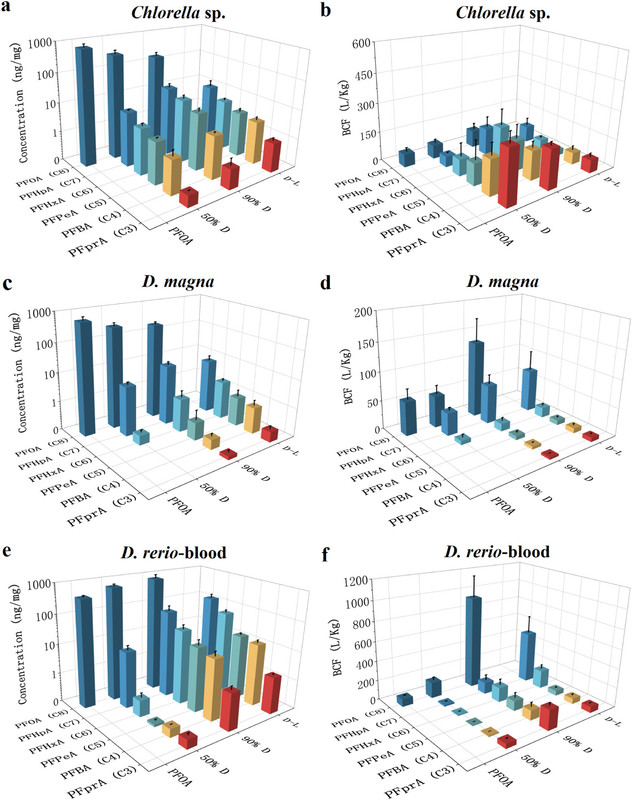
The caption:
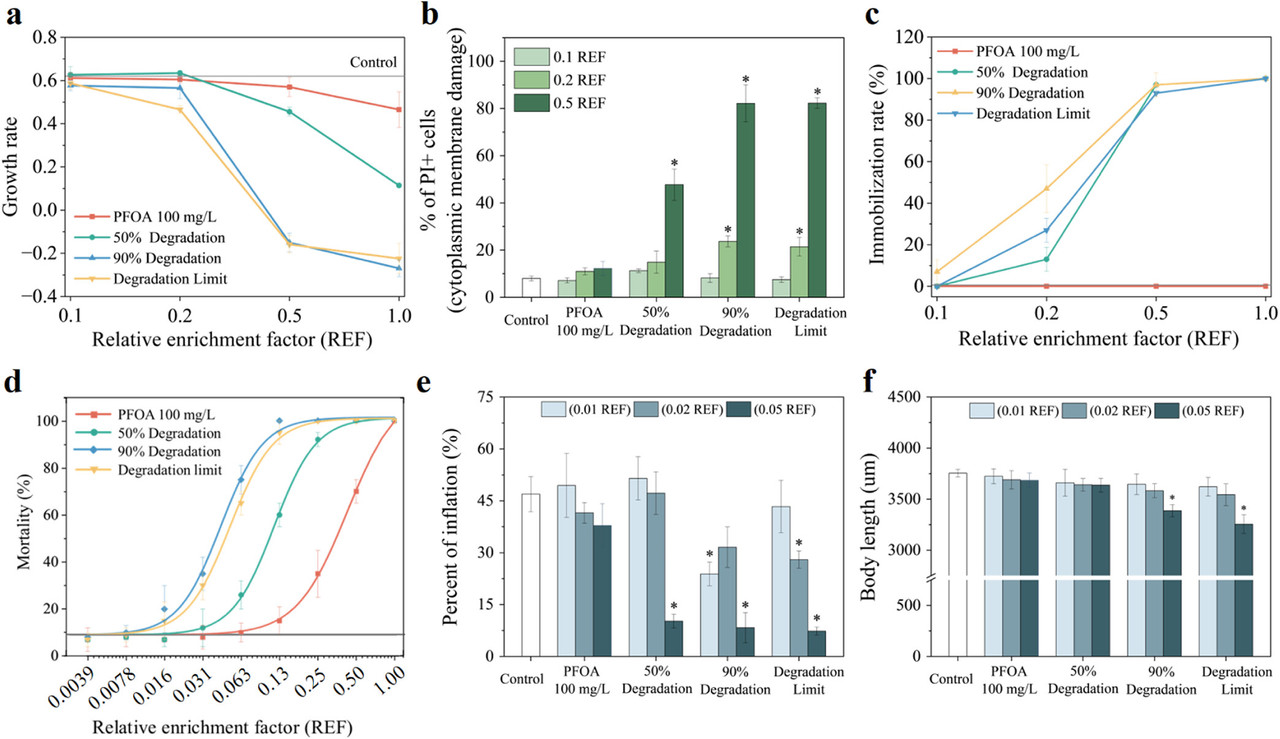
The caption:
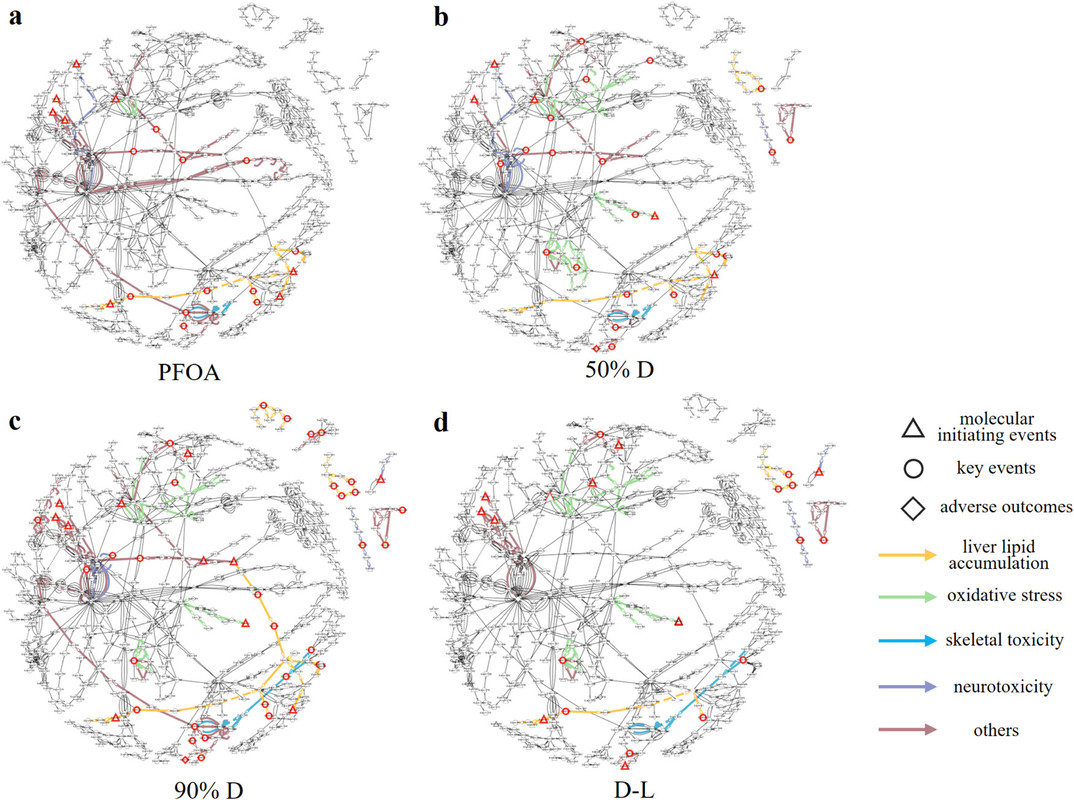
The caption:
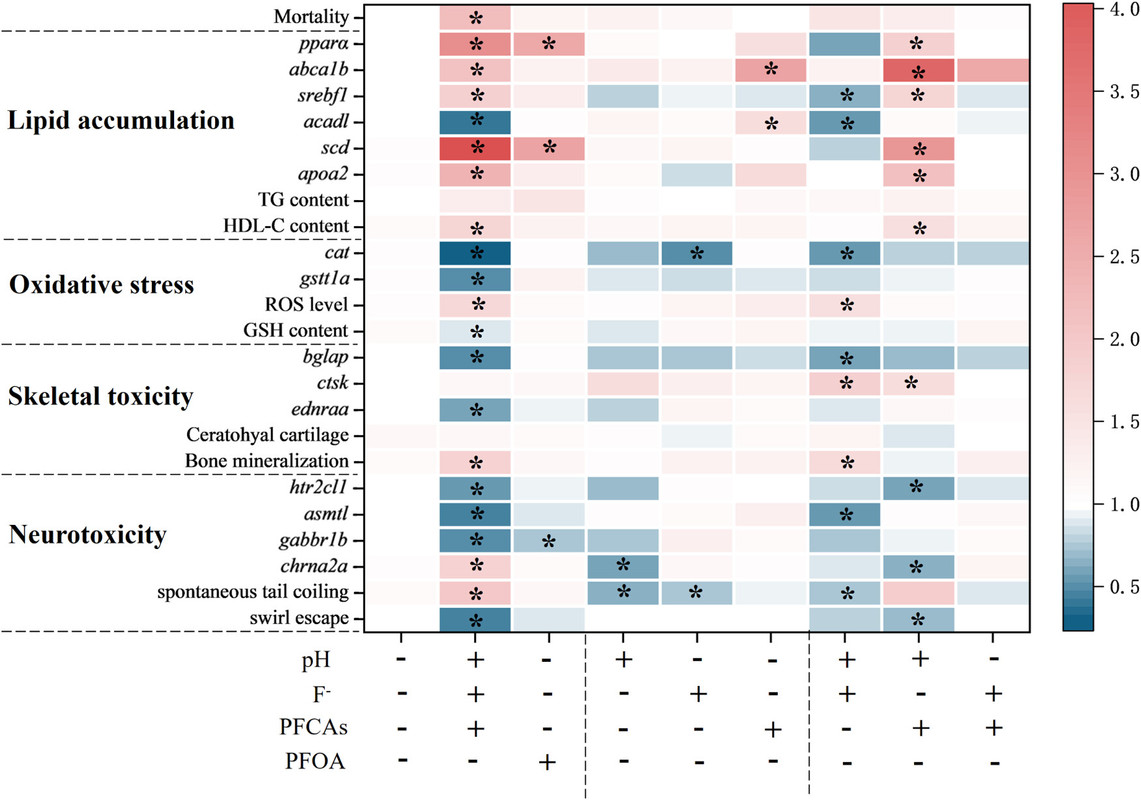
The caption:
Ionizing radiation, as indicated above, is a sink for perfluoroorganics.
In general, the shorter chain acids are more difficult to mineralize; worldwide, the concentration of trifluoroacetic acid is rising in the environment.
I have suggested to my son, who in his career will probably have access to used nuclear fuels, that this might be addressed by assuring that highly radioactive solutions utilized for the mineralization of trifluoroacetic acid should remain highly basic, resulting in radical decarboxylation, ideally under conditions in which metastable trifluoromethanol is formed, decomposing ultimately to fluorophosgene, which reacts in basic water to give carbonate and fluoride salts.
A note: We have here at DU the fossil fuel industry working, as it has successfully done for generations, thus leaving the world in flames, attacking nuclear energy while working to rebrand fossil fuels as "green hydrogen" in an ethically despicable marketing scheme. One lie among many is that hydrogen fuel cells are "green." The solid electrolytes in many hydrogen fuel cells are fluoropolymers, members of the same class of fluoroorganics that have become a major source of pollution. (Teflon is a fluoropolymer, and, yes, it is an environmental problem.) Happily, for generations, hydrogen fuel cells have been a line of bullshit along with all conceptions of a putative "hydrogen economy," which has not happened, is not happening, and will not happen. The purpose of the bullshit is to keep the fossil fuel industry afloat and rich, something it clearly is.
The fossil fuel industry engaged in this particular fraud, likes to carry on about so called "nuclear waste," despite the fact that valuable used nuclear fuels have a spectacular record of not killing anyone, certainly nowhere near the seven million people who die each year from the combustion of fossil fuels and biomass. About 3% of the world's fossil fuels are consumed to make hydrogen. In fact, the storage of used nuclear fuel over a period of 70 years has not killed as many people as will die in the next 12 hours from fossil fuel waste, aka air pollution, about 9500 people, meaning at 3%, close to 300 of those deaths will be for the purpose of making hydrogen. This said, captive hydrogen is a necessary industrial product used to make fertilizers, without which the majority of the world population would starve to death. The idea of hydrogen trucks, cars, helicopters, lawn mowers and mechanical toothbrushes is abysmally stupid.
The fossil fuel industry attacks nuclear energy because nuclear energy represents the only primary energy route capable of driving it out of business.
Hydrogen, despite marketing by the fossil fuel industry to the contrary, is not widely used as a fuel, nor should it be, since it has horrible physical properties that make it dangerous, uneconomic, and dirty, particularly given its source and the thermodynamics of producing it.
Hydrogen fuel cells, from all of the above, are even worse than hydrogen trucks, cars, helicopters, lawn mowers and mechanical toothbrushes by direct combustion.